Atmospheric Surface Modification Of Polymers For Biomedical Device Adhesion
Abstract
Biomedical applications require polymers that react properly with the biological environment in which they are employed. Since biocompatibility involves the interface between the device and the biological environment, surface modification techniques can be critical to solve adhesion issues, avoiding costly changes of materials. Atmospheric surface modification techniques such as air plasma, flame plasma and chemical plasma discharges can positively affect medical polymers such as high molecular weight polyethylene in a localized manner to produce useful results, such as increasing the hydrophilic nature of the surface or crosslinking functional groups to the surface. An in-depth examination of the design of these atmospheric technologies relative to specific biomedical materials and applications is presented.
Introduction
Plastics, polymers, and resins have become widely accepted for in vivo and in vitro medical applications. Many of these materials have properties that lend themselves well to the manufacture of medical appliances or devices; they are relatively inexpensive and easily molded or formed into complex shapes, and bulk physical properties may be selected from a wide range of parameters such as rigidity and temperature stability. Unfortunately, fabrication procedures that require bonding are difficult to achieve, and biological interface reactions within the body or in the laboratory can limit their in vivo and in vitro performance.
Atmospheric pressure plasma technology offers a new technique for easing these limitations by modifying the surfaces of these polymers. By altering just the first few atomic layers, the surfaces of most medical polymers can be rendered wettable so that adhesive bonding can be achieved to troublesome materials such as polyolefins, silicones, and fluoropolymers. In a similar fashion, more exotic processes such as plasma grafting and polymerization can produce totally new custom surfaces without loss of the desirable characteristics of the bulk material.
Vacuum, low pressure plasmas have traditionally been used in the aerospace, semiconductor, and electronics industries for more than 30 years for cleaning, etching, and surface treatment of various materials. Like vacuum plasmas, atmospheric plasma treatments do not affect the bulk of the materials and plasma treated parts are generally visually and physically indistinguishable from untreated parts.
Plasmas are now routinely used for controlling the wettability of test tubes and lab vessels, for pre-bonding preparation of angioplasty balloons and catheters, for treating blood filtration membranes, and to manipulate surface conditions of in vitro structures to enhance or prohibit culture cell growth.
Given enough energy, any gas can be excited into a “plasma,” which is a mixture of ions, electronics, excited species, and free radicals. There are many temperature and pressure conditions where this phenomenon will occur. But for practical considerations dielectric barrier discharge plasmas are commonly generated at radio frequency, enabling these processes to take place at low temperatures (25-100ºC) and atmospheric pressure where surface reactions with polymers are feasible without bulk interactions.
Plasma “treatment” usually refers to a plasma reaction that either results in modification of the molecular structure of the surface or atomic substitution. Even with benign gases such as oxygen or nitrogen, plasma treatment can create highly reactive species at low temperatures. High energy ultraviolet light is emitted in the process which, along with the high energy ions and electrons, provide the energy necessary to fracture polymer bonds and initiate chemical reactions at the surface. Only a few atomic layers on the surface are involved in the process, so the bulk properties of the polymer remain unaltered by the chemistry while the low process temperature eliminates concerns about thermal modification or distortion of the bulk. Unique reactions can be promoted by appropriate choice of reactant gases, and unusual polymer byproducts and structures can be formed.
In many instances, plasma cleaning with benign gases such as oxygen or nitrogen provides adequate surface activation for enhanced wetting and adhesive bonding. With other targeted end
results or substrate materials, it may be necessary to utilize reactants which result in “grafting” or surface chemistry modification to achieve the desired results.
It is frequently possible to select reactants that form volatile byproducts upon reaction of the plasma with the substrate material. These, upon desorbtion from the surface of the treated material, are removed by an exhaust system, resulting in etching of the surface without the necessity for further scrubbing or neutralizing.
Biomedical Device Applications
Plasma treatment of polyethylene or polypropylene disposable Petri or Assay dishes greatly enhances wetting. Contact angles as low as 22º have been demonstrated on these materials after brief oxygen plasma exposure (see Table 1). When these parts are properly packaged after treatment, the contact angle has been seen to be stable for several years.
Conversely, many medical polymers can be made extremely hydrophobic. Teflon-like films and other similar surface treatments can be easily accomplished on most polymers using fluorinated gases. For example, small diameter tubes can be treated so that when immersed in aqueous solutions they do not draw fluid by capillary action.
One of the simplest techniques used to evaluate plasma surface treatment is a wetting angle test using a contact goniometer. Surface roughness and substrate cleanliness need to be tightly controlled to obtain quantitative data. Standard wetting solutions are often used to obtain accurate surface energy values.
Most untreated polymers are only poorly wettable. Initial contact angles may vary from 60-100º. Table 1 shows a sample of some typical contact angle measurements:
Table 1.
Typical wetting angles, before and after Oxygen plasma treatment.
| Before | After | |
| Polypropylene | 87° | 22° |
| Polyethylene | 87° | 22° |
| Polyamide (nylon) | 73° | 15° |
| Polyimide | 79° | 10° |
| Polycarbonate | 75° | 33° |
| Tefzel | 92° | 53° |
| PFPE | 96° | 68° |
Many intravascular devices, such as balloon catheters, are assembled by adhesive bonding of polyethylene components. Chemical surface activation or mechanical surface roughening techniques provide only modest bonding performance, with bond failures noted after as few as eight repetitive inflations. With plasma treatment, up to 40 repetitions are achievable. Typical bond strength data are shown in Table 2.
Table 2.
Typical lap-shear bond strengths (psi), without and with plasma treatment.
| Without | With | |
| Polypropylene | 370 | 1380 |
| Polyethylene (low density) | 370 | 1450 |
| Polyethylene (high density) | 315 | 3125 |
| Nylon | 850 | 4000 |
| Polystyrene | 570 | 4000 |
| Polycarbonate (Lexan) | 410 | 928 |
| Tefzel | 410 | 3200 |
An oxygen plasma not only removes organic residues but also chemically reacts with the surface to form strong covalent carbon-oxygen bonds, which are much more polar and more reactive than the initial carbon-hydrogen bonds. The increased polarity of the surface accounts for the substantial increases in wettability and adds a degree of covalent bonding to the surface- adhesive interface. (Note that other gases may be used to attain similar results in instances where oxidizing species may be harmful to components of the assembly.)
The bond strength ultimately realized will certainly be affected by:
- Initial cleanliness of the surface(s).
- Wetting of the surface by the adhesive.
- Cross-linking effects.
- Chemical interaction of the adhesive with the surface.
Any mold release compounds, unpolymerized monomers, plasticizers, or additives that may have migrated to the surface must be removed either by plasma cleaning or washing before surface modification is attempted. Immediate assembly is usually advised after the surface has been prepared. Once the surface has been optimized and bonded, the bond is permanent and does not degrade over time.
Biomedical Plastics & Adhesives
The strongest advances in plastics usage involve engineered resins such as polycarbonate and thermoplastic elastomers, which have favorable cost-performance benefits. Polypropylene, polyethylene, polyurethane and polyvinyl chloride retain leading positions in non-invasive medical products and standard medical packaging due to their low cost and amenability to radiation sterilization.
Polypropylene, like polyethylene, is a crystalline thermoplastic which has excellent thermal and chemical resistance properties and high moisture resistance. Polypropylenes are specifically often selected for their good mechanical properties, but they are a difficult-to-bond substrate since they feature no surface functional sites or inherent surface roughness to which an adhesive can secure itself. Polypropylenes are also characterized by either linear or branched carbon chain polymers, low surface energies, low porosity, and non-polar surfaces. Polyethylene, and particularly low density polyethylene, is a semi-crystalline thermoplastic produced by way of a free-radical- polymerization reaction. Although this polyolefin typically has lower strength and hardness properties, it offers numerous benefits including flexibility, clarity, and enhanced impact and stress cracking resistance. High-density polyethylene is similar to low-density polypropylene in the polymerization process used to obtain the resin. However, the density of the polyethylene increases, resulting in higher strengths, increased hardness, and enhanced chemical and abrasion resistance.
Hard-to-bond plastics such as polyolefins are most often assembled using adhesives. While adhesives are the most versatile assembly method for plastics, only a few industrial adhesives offer suitable bond strengths on hard- to-bond plastics. Cyanoacrylate, light-curing cyanoacrylate, hot-melt and light-curing acrylic adhesives have typically been used with typical difficult-to-bond plastics. Acrylic adhesives are also being introduced for use with hard-to-bond plastics.
Cyanoacrylate adhesives are polar, linear molecules that undergo an anionic polymerization reaction. A weak base, such as moisture, triggers the reaction causing the linear chains to form. Many cyanoacrylate formulations are available with varying viscosities, cure times, strength properties and temperature resistance. Cyanoacrylates form thermoplastic resins when cured. Standard unfilled ethyl monomer based cyanoacrylates typically exhibit low impact and peel strengths, low to moderate solvent resistance, and maximum operating temperatures of 160-180 degrees F. They will fixture in as little as three seconds. Rubber-modified cyanoacrylate formulations offer improved peel and impact strengths over standard cyanoacrylates. The compounded rubber added to ethyl formulations slightly increases fixture time to 30 seconds to two minutes. Thermally resistant ethyl cyanoacrylates can withstand continuous exposure to test temperatures up to 250 degrees F. Light-curing acrylics cure by way of a free radical reaction to form thermoset resins when exposed to light of the appropriate wavelength and intensity. Light-curing cyanoacrylates are ethyl-based products that have photoinitiators added to the formulation, allowing them to fixture rapidly on exposure to low-intensity light, and to cure in shadowed areas. Physical performance characteristics of these adhesives are similar to those of traditional cyanoacrylates. Polyolefin hot melts provide good resistance to moisture as well as excellent resistance to polar solvents, acids, bases and alcohols, offering superior adhesion to polypropylene when compared to other types of hot melts. Reactive urethane hot melts perform well on hard-to-bond plastics, processing at temperatures of approximately 250 degrees F which is as much as 200 degrees F cooler than EVA, polyamide, and polyolefin hot melts.
Descriptions of Equipment & Processes
Release agents and other contaminations on molded and formed medical parts, however, can impede the performance of these adhesives dramatically if they are not addressed with surface pretreatments such as air plasma, flame plasma or atmospheric chemical plasma.
An atmospheric air plasma treating system consists of two major components, a power supply and treatment station. The power supply accepts standard utility electrical power and converts it into single phase, higher frequency power that is supplied to the treating device. The treating device applies this power to the surface of medical plastics through an air gap, via an electrode design. When air is exposed to different voltages, an electrical discharge develops. When this occurs, neutral molecules and electrically charged molecules collide. These collisions cause neutral molecules to become electrically charged, resulting in filamentary discharges or “streamers”. Such filamentary discharges create a cloud of ionized air
– or an “air plasma”. When a medical plastic surface is placed under an air plasma discharge, electrons bombard the treatment surface with energies two to three times that necessary to break the molecular bonds on the surface of most substrates. The resulting free radicals react rapidly with other free radicals on the same or different molecular chain, resulting in cross-linking. Oxidative affects on treated surfaces increases surface energy as a result of polar groups being created on the surface, primarily in the form of hydroxyl groups, carbonyl groups, amide groups and carboxylic acid. Electron and ion bombardment will also create a cleaning effect on surfaces of medical plastics. Since exposures of treated surfaces to high levels of ambient humidity and temperature accelerates polymer side chain mobility and treatment degradation, it is recommended that down-stream plastic decoration take place directly following treatment.
Atmospheric flame plasma systems are comprised of a combustion/electrical station and a burner assembly, manufactured with two primary burner configurations – ribbon and enhanced velocity. A flame plasma is formed when a flammable gas and atmospheric air are combined and combusted to form an intense blue flame. The surface of medical plastics are made polar as species in the flame plasma affect the electron distribution and density on the surface. Polar functional groups such as ether, ester, carbonyl, carboxyl, and hydroxyl are contained in a flame plasma; these are incorporated
into the surface and affect the electron density of the polymer material. This polarization and functionalization is made through reactive oxidation of a surface. ESCA analysis shows that oxidation depth through flame treatment is 5- 10nm. This is generally less in depth than air plasma treatment, where oxidation depth is believed to be over 10nm. However, flame plasma treatment’s extensive oxidation, due to reactions with OH radicals in the flame, results in a cleaned and highly wettable surface which is relatively stable upon aging.
An atmospheric chemical plasma system is also composed of a power supply and treatment station whereby the system generates an electrically charged atmosphere similar to air plasma, but uses chemical atmospheres in place of air to introduce a wide range of surface modifications to a substrate. The systems are characterized by their generation of high density reactive species for low temperature material processing. The chemical plasma process can involve surface preparation via the breakdown of low molecular weight organic materials (LMWOM) and surface decontamination, fine etching of the surface to create new topographies, grafting of new functional groups or chemical species on the surface, and the deposition of coatings on the surface. The treatment process is designed to allow the interchange of gas chemistries relative to the application requirements. In the case of medical plastic parts, loose surface oligomers and other residues are repetitively cleaved and degraded until they are removed largely by a combination of bombardment by ions and electrons. These organic residues are converted into water vapor, carbon dioxide, and other nontoxic gases or volatilized materials. A noble carrier gas such as argon is typically used to initiate the breakdown of LMWOM and create surface etching for greater adhesion of adhesives. The gas species which is ionized, along with the composition and structure of the polymer itself, are the key factors that determine the degree of etching. To maximize adhesion of adhesives on plastics (particularly thermoplastic olefins), the deposition of polar functional groups from the use of oxygen and acetylene reactive gases with the carrier gas can be particularly effective. Atmospheric chemical plasma treatment systems are typically non-thermal, atmospheric-pressure, glow-discharge plasma systems which generate uniform and homogenous treatments. The level of surface tension and longevity of treatment are both typically greater than air plasma treatment effects, and similar in treatment effect to flame plasmas. The medical plastic is optimally treated by atmospheric chemical plasma when it is positioned several millimeters downstream from the source. Line speed, power level, chemistry, chemistry mixtures and material composition primarily determine levels of etching and functionalization which can be achieved.
Application of Equipment and Processes
It was theorized the application of atmospheric air plasma and flame plasma surface pretreatment would sufficiently clean the surface of polypropylene, polyethylene and polyurethane – based medical plastic parts of LMWOM following molding and forming processes. If accomplished, this surface improvement would be identified through surface tension testing using ethyl cellosolve / formamide (dyne) solutions, and subsequently through adhesive adhesion testing. As such, an atmospheric air plasma device (Figure 1) was employed with an internal air blower to discharge a blown arc at 480W. The performance of this device was compared with a flame plasma device (Figure 2) with compressed air and methane inputs. The stoichiometric ratio air/gas ratio was maintained at 10:1 to create a thermal discharge of 11,282 BTUs/hr.
Medical device manufacturers provided common plastic part assemblies for conveyance beneath each of the treatment devices at industry-standard discharge gaps between treatment device head and substrate to achieve optimum electron bombardments.
Presentation of Data & Results
Trial pretreatment conditions and results are shown in Table 3. Results presented indicate a final dyne, or surface tension, at which minimum adhesive adhesion was achieved relative to assembly requirements specified by the medical device manufacturers supplying these materials.
Relative to conditions, it is noted that the airflow of the atmospheric air plasma device optimized treatment at approximately twice the airflow of the flame treatment device, and the dwell time of air plasma treatment was ten times that of flame plasma. Although this is the case, the high velocity and thermal output of the flame plasma device created the necessity to distribute this output at a higher (optimized) speed so as not to affect the surface morphology of the trial materials. This
optimized speed met all medical device manufacturer’s process requirements.
Interpretation of Data
It is evident from the trial data that flame plasma effected a higher level of cleaning and surface tension compared to atmospheric air plasma through higher electron bombardment, increased surface electron density, and high surface oxidation.
The primary effect of the enhanced velocity discharge burner relative to the flame plasma device is interpreted to be related to 1) increased treatment (bombardment) efficiency, 2) greater power density transfer, and 3) a more homogeneous discharge profile.
Results and Discussion:
Plastics usage in the healthcare field encompasses several distinct markets, and predominantly involving applications for medical devices, biocompatible implant devices and for medical packaging. Polypropylene, polyethylene, polyurethane, polyethylene oxide, styrene-acrylonitriles, polysulfones, liquid crystal polymers, and polyvinyl chloride were exposed to pre-treatment by plasma discharge technologies to improve adhesion relative to non-invasive biomedical products and standard medical packaging due to their low cost and amenability to radiation sterilization. Invasive biocompatible / biodegradable plastics such as aliphatic polyesters, PEG based polymers, PVA and copolymers, natural polymers and polyanhydrides have also shown surface adhesion enhancement by plasma discharge technologies.
Release agents and other contaminations on molded and formed medical parts were found to impede the performance of medical adhesives dramatically if they were not addressed with surface pretreatments such as air plasma, flame plasma or atmospheric chemical plasma.
Results presented indicate a final dyne, or surface tension, at which minimum adhesive adhesion was achieved relative to assembly requirements specified by the medical device manufacturers supplying these materials. Flame plasma effected a higher level of cleaning and surface tension compared to air plasma through higher electron bombardment, increased surface electron density, and high surface oxidation.
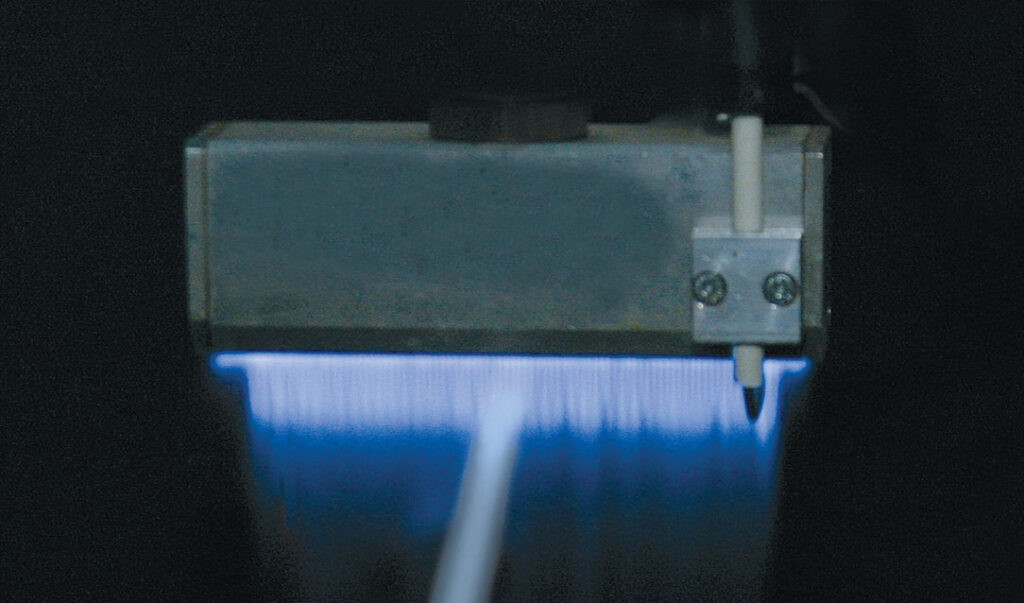

Table 3. Trial Treatment Chart
| Air Plasma | Flame Plasma | |||
| Material | Initial Dyne | Final Dyne | Initial Dyne | Final Dyne |
| PP | 30 | 44 | 30 | 46 |
| PE | 31 | 44 | 31 | 46 |
| PU | 31 | 40 | 31 | 42 |
| Treat Width | 50.8mm | 150mm | ||
| Airflow | 311lpm | 150mm | ||
| Treat Gap | 6.35mm | 41.3mm | ||
| Ave. Speed | 10fpm | 100fpm | ||
Conclusion
The use of low polarity polyolefins in the manufacturing of medical device assemblies such as catheters, syringes, tubings and other components may be more suitably cleaned of molding and forming organic contaminations and functionalized using flame plasmas as opposed to atmospheric air plasmas for improved adhesive adhesion.
References:
- Salerni, C., Adhesives & Sealants Industry, “Adhesive Technologies for the Assembly of Hard-to-Bond Plastics” (2003).
- Jones, H.R.N. (Howard Richard Neil), “The Application of Combustion Principles to Domestic Gas Burner Design”, E. & F.N. Spon Ltd.(1989).
- Mittal, K.L., and Pizzi, A., “Adhesion Promotion Techniques: Technological Applications”, Marcel Dekker (1999).
- Brewis, D.M. and Mathieson, I., “Adhesion and Bonding to Polyolefins”, Rapra (2002).
- J.R. Hollohan and A.T. Bell, Techniques and Applications of Plasma Chemistry, John Wiley & Sons, New York, NY, 1974
- Chapman, Glow Discharge Processes, John Wiley & Sons, New York, NY, 1980
- T.J. Hook, J.A. Gardella, and L. Salvati, Journal of Materials Research, 2, 132,
1987
- D.S. Everhart and C.N. Reilly, Annals of Chemistry, 53, 665, 1981
- M. Szycher and W.J. Robinson, Editors, Synthetic Biomedical Polymers, Technomic, Westport, CT, 1980
- R. d’Agostino, Editor, Plasma Deposition, Treatment, and Etching of Polymers, Academic Press Inc., San Diego, CA, 1990
- Jim Scheppele, “Surface Modification of Plastics for Medical Device Applications”; http://www.a2c2.com/archive/699surfacemod.htm


